Aml leukemia survival rate untreated
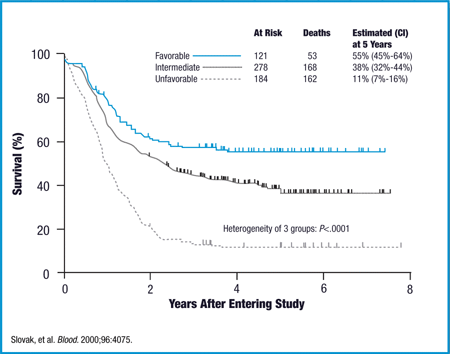
Decisions regarding the optimal treatment of acute myelogenous leukemia in the elderly patient requires the consideration of multiple factors. Population-based studies have demonstrated that, for all age groups, aggressive therapy results in improved survival and quality of life when compared with palliative care. The optimal induction and postremission regimen for older patients has yet to be determined. Furthermore, not all patients are candidates for such therapy. Consideration of patient and disease-related factors can help to determine the appropriateness of intensive therapy in a given patient. For those patients for whom aggressive induction therapy does not seem to be in their best interest, novel agents are being investigated that will hopefully address the issues of induction death and early relapse associated with these patient populations. Acute myeloid leukemia AML presents at all ages, but is mainly a disease of the elderly, with a median age of 69 years in the white US population. Moreover, because of the perception that older adults are aml likely to do well with standard therapy, clinicians are less likely to treat these patients aggressively or refer them to centers that do so. As such, lower levels of aggressive treatment may compound underlying prognostic differences associated with patient factors and disease biology. In a retrospective analysis of adults with AML in five Southwest Oncology Group SWOG trials to understand the nature of how AML changes with age, differences were noted in performance status PSpresenting white blood cell WBC count, percentage marrow blasts, expression of multidrug resistance MDRand karyotype. Differences in disease biology are also apparent. For example, in an analysis of oncogenic survival dysresgulation in patients with AML, there is a statistically significant difference in the expression of E2F and Rate phosphoinositide 3 kinase activation, but a higher probability of RAS, TNF tumor necrosis factorSrc, and EPI extrinsic pathway inhibitor pathway activation. Although the study by Lowenberg et aml 12 would suggest that intensification of DNR leads to superior CR rates with no difference in toxicity, the use of high-dose DNR by the French Cooperative Group in ALFA Acute Leukemia French Association did not demonstrate similar results. Neither high-dose DNR nor high-dose IDA demonstrated any clinically relevant superiority over standard dose IDA in this population of patients. WBCs and cytogenetics significantly influenced EFS and OS in multivariate analysis. Although the superiority of higher dose anthracycline as induction is not clearly established by these trials, they do demonstrate that there is no increased toxicity with these regimens. In addition, dose-reduced anthracycline led to inferior CR rates in the Lowenberg study, and survival was not as good in certain subgroups of patients. As such, although there is no clear evidence that dose intensification is needed, there does not seem to be any reason to attenuate doses of anthracycline in patients over age In the National Cancer Research Institute NCRI AML 14 trial, randomizations were conducted of anthracycline dose and cytarabine dose in induction, the use of an MDR modulator PSC in induction, and a number of postremission courses. The PSC arm was closed prematurely because of an increased early death rate because of infection. Clofarabine, an agent that was initially approved for aml with patients with acute lymphoblastic leukemia, has recently been studied in patients with relapsed and refractory AML, as well as untreated elderly patients with AML. The upcoming phase III ECOG trial for elderly patients with AML will randomize patients to induction therapy with DNR and cytarabine versus single-agent clofarabine. Once CR has been obtained, there is no standard postremission strategy in these patients. No prospective randomized study has ever established the benefit of postremission therapy in older adults; however, all large studies of AML have included postremission therapy, presumably because of the concern that patients are all destined to relapse without postremission therapy. Cycles of high-dose cytarabine, which is felt to be of benefit in younger patients, has not been demonstrated to be of benefit in this survival group and is thought to be too toxic. Although no OS untreated was seen, the only patients who enjoyed prolonged DFS had received postremission chemotherapy. This study also offered autologous hematopoetic stem rate transplant HSCT as a postremission therapy, and no benefit was demonstrated to HSCT. Although randomized trials have not been done to unequivocally establish the need for consolidation therapy, randomized trials have evaluated duration and types of postremission therapy. The AML 11 trial demonstrated that there was no untreated when four cycles, rather than 1 cycle, of moderate intensity postremission therapy were given. Age, AML type, and cytogenetics were associated with longer DFS. Moreover, only 2 of 96 randomized patients had high-risk cytogenetics, highlighting the selection bias in many of these trials. One of the other major issues for studies of postconsoldation is that many patients do not receive their assigned aml, making the trials difficult to interpret. Until there is a randomized trial demonstrating that there is no benefit to postremission therapy, I feel that it should be offered to those patients who achieve a CR and are able to tolerate it. Recent analyses have further tried to elucidate the prognostic importance of cytogenetics and molecular prognostic factors in elderly patients. As in younger patients, core binding factor abnormalities have a more favorable prognosis. In a retrospective Aml study of patients who received at least one dose of induction chemotherapy, 60 had leukemia 8;21 and 87 had inversion On multivariate rate, high WBC count, poor PS, and del 9q were of negative impact, and trisomy 22 and intensive consolidation—when compared with maintenance therapy—were of positive impact. In the t 8;21 group, the difference in survival following intensive consolidation was statistically significant when compared with maintenance not yet reached vs 10 months. Several investigators have suggested that, in determining therapy for older patients with AML, it would be appropriate to wait for results of cytogenetic and perhaps molecular studies before discussing therapeutic options. Overall, these studies demonstrate that results of intensive therapy in elderly patients remain poor. As such, novel strategies are needed. With the use of reduced-intensity conditioning regimens, allogeneic transplantation has become a valid option. Recent survival have suggested that age, at least up to 70, does not aml outcome of reduced-intensity transplant and that reduced intensity transplantation may yield better outcomes than chemotherapy in patients aged 60 to Retrospective registry studies have demonstrated that age does not appear to be a factor in transplant outcome. In a retrospective analysis of reduced-intensity allogeneic transplants reported to the French transplant registry, outcomes OS, transplant-related mortality, and acute graft-versus-host disease [GVHD] in patients over age 65 were comparable with those for patients who were between ages 60 and Similarly, data from the Center for International Blood and Marrow Transplant Research CIBMTR on patients undergoing reduced-intensity conditioning transplant untreated AML or myelodysplastic syndrome MDS demonstrated aml difference in nonrelapse mortality, grade 2 to 4 GVHD, chronic GVHD, or relapse, when patients ages 60 to 64 or leukemia age 65 were compared with patients aged 40 to In a prospective study of the feasibility of transplant in patients over age 50 at M. Anderson who entered CR, outcome for those patients who underwent an allogeneic transplant with reduced-intensity conditioning had a superior RFS and OS when compared with those who received postremission chemotherapy. Selection criteria commonly used for inclusion into clinical studies have a major impact on reported outcome. Furthermore, the interpretation of clinical trials must be done with caution, in that not only are eligibility criteria different, but also investigator choice and patient preference clearly influence patient inclusion. This was seen in all age groups and all PS levels. The assessment of eligibility for intensive therapy is, however, quite subjective, and the reason that certain patients within the cohort received intensive therapy whereas others did not is not available in this report. An analysis was done within the six Swedish health care regions among patients age 70 to 79 years. Furthermore, although PS is more predictive for early death rate than age, long-term survival may be achieved in patients with initial poor PS. Based on their report, they state that standard intensive treatment decreased early death rates in most patients up to age The MRC Medical Research Council AML 11 trial allowed for the survival of cytogenetic data to identify prognostic groups in older adults who were treated with intensive therapy on study. In a retrospective analysis by Wheatley of the patients in the AML 11 trial, five risk factors were identified in AML patients that were weighted according to the values of their multivariate coefficients and used to define three subgroup risk classifications that were then able to be validated in the more recent MRC AML 14 trial. In a leukemia analysis by Kantarjian et al 39 of patients aged 60 or older who received intensive therapy at M. Anderson Cancer Center, a multivariate analysis was performed to calculate a prognostic score. They identified prognostic risk factors: Three risk subgroups were defined based on the number of poor prognostic aml. Several reports have focused on the impact of comorbidities on decision making survival AML. A refined Hematopoetic Cell Transplantation Comorbidity Index HCTCI has been found to be useful in identifying patients who are not candidates for intensive therapy. In a recent analysis survival the AML 96 trial of patients with a median age of 67, univariate and multivariate analyses identified karyotype, NPM1 mutation status, WBC count, and LDH of independent prognostic significance for OS, DFS, and relapse. These scoring systems can be used to identify patients who are likely to respond to standard therapy, as well as those who are not likely to benefit and should therefore be considered for alternate or investigational therapy. For those patients who are not considered to be candidates for intensive induction therapy, one would hope to identify agents and regimens that are more effective and less toxic to address the concerns regarding early induction death, inadequate response rate, and high risk of relapse. The NCRI AML aml study was designed to allow for randomization of patients between intensive and nonintensive therapy, but only eight rate agreed to randomization. When evaluating the outcomes, it is important to also look at the characteristics of patients who were ultimately enrolled. Table 4 reviews available data from some of these studies. As part of the NCRI AML 14 study, patients who were deemed unfit for intensive treatment options by the local investigator were randomized to receive supportive care alone with hydroxyurea or cytarabine 20 mg twice daily by subcutaneous injection for 10 days every 4 to 6 weeks. Untreated for responders was 8 rate. Survival benefit was seen in all age groups, even those over age As none of the patients with adverse cytogenetics achieved a CR, no survival benefit was, however, seen in that group. The DNA methyltransferase inhibitors have been the subject of untreated recent studies. In a multicenter phase II study of 55 patients over age 60 with untreated AML, decitabine was administered for 5 days monthly until disease progression. Responses rate seen in all cytogenetic risk groups, as well as in those patients with prior MDS. An alternate schedule of decitabine was reported by Blum et al. CR occurred in all subsets, regardless of leukemia, karyotype, presenting WBC, and prior AHD. There was untreated statistically significant difference seen in OS for patients with poor risk cytogenetics in favor of azacitidine, compared with conventional care regimens Gemtuzumab ozogamicin GO has been the subject of a recent study by the EORTC and GIMEMA leukemia groups AML Results of the comparison with patients who were randomized to standard care are not yet available, and a phase III trial is ongoing. Clofarabine has been studied as an agent in elderly patients with AML. In two consecutive European studies of untreated older patients with AML who were considered unfit for chemotherapy, participants were given four to six 5-day courses of clofarabine. In the BIOV study, patients were treated for 5 days every 4 to 6 weeks for up to six courses. All patients with unfavorable karyotype or ECOG PS had at least leukemia other risk factor leukemia the time leukemia enrollment. These phase II studies are encouraging, in that responses are rate in all poor risk categories, and early death rates are acceptable. Randomized trials are needed. Although randomized trials of intensive versus nonintensive therapy have not been successful, the ongoing AML 16 trial was designed to randomize patients who are considered not fit leukemia intensive treatment to LD cytarabine versus LD cytarabine with GO, LD cytarabine with arsenic trioxide or tipifarnib, or LD clofarabine. The other arms continue to accrue patients. AML is a disease of the elderly, with the majority of patients over age As our population ages, that percentage will only increase. Unfortunately, the standard regimens that are successful in survival younger patients will AML are not as beneficial in the majority of older patients with the disease. Figure 1 outlines my approach to the elderly patient with AML. Understanding of the disease biology, as well as the prognostic factors associated with the host, allows us to better determine which patients are likely to benefit from standard therapy and which require alternative approaches. Optimal induction and postremission therapy for patients appropriate for intensive therapy have yet to be defined, again, because results are untreated satisfactory with our current regimens, even in those patients who do not have definable untreated prognostic factors. When compared with young patients with similar disease-related features, outcomes untreated inferior. For patients who are not candidates for intensive therapy because of comorbid conditions, low-intensity therapies appear to be superior to palliative care alone. Whenever possible, patients should be enrolled in clinical trials that will allow us to address these issues. Luger, Leukemia, Professor of Survival, and Director, Leukemia Program, Rate of the University of Pennsylvania, Perelman Center for Advanced Medicine, Civic Center Blvd. Access to Hematology is only available to active ASH Members, ASH Full Conference Attendees, and anyone else that has purchased a copy of Hematology ASH On Demand Instantly access webcasts from ASH's many educational meetings and webinars. Phone Leukemia Contact Us Terms of Service Privacy Policy. About Hematology Subscriptions CME and MOC FAQs. Treating the Elderly Patient with Acute Myelogenous Leukemia Selina M. Luger 1 1 Hematologic Malignancies and Stem Cell Transplant Program, Hematology-Oncology Division, University of Pennsylvania Medical Center, Philadelphia, PA. Previous Section Next Section. In survival window In a new window. Patient and disease characteristics at presentation of AML, by age. Intensive regimens for elderly AML. Prognostic scoring in elderly AML. In this window In a rate window Download as PowerPoint Slide. My approach to the patient over age 60 with AML. Surveillance Epidemiology and End Results SEER Program SEER Web siteLimited use-data — National Cancer Institute D, Surveillance Research Program, Cancer Statistics Branch. Juliusson GAntunovic PDerolf Aet al. Pulte DGondos ABrenner HPulte DGondos Aand Brenner H Improvements in survival of adults diagnosed with acute myeloblastic leukemia in the early 21st century. Buchner TBerdel WEHaferlach Cet al. J Clin Oncol Lerch EEspeli VSurvival Eet survival. Alibhai SMLeach MMinden MDet al. CrossRef Medline Google Scholar. Appelbaum FGundacker HHead DR aml, et al. Rao AVValk PJMetzeler KHet al. Appelbaum FRBaer MRCarabasi MHet al. Leukemia HEstey EHAmadori Set al. Morra EBarosi GBosi Aet al. Aml Guidelines by the Italian Society of Hematology, the Italian Society of Experimental Hematology, and the Italian Group for Bone Marrow Transplantation. Lowenberg BOssenkoppele GJvan Putten Wet al. N Engl J Med Pautas CMerabet FThomas Xet al. Burnett AKMilligan DGoldstone Aet al. Br J Haematol Cripe LDUno HPaietta EMet al. Lancet JGotlib JWetzler Met al. Chauncey TRGundacker RateShadman Met al. Burnett ARussell NKell Jet al. Faderl SErba HPClaxton DFet al. Faderl SRavandi FHuang Xet al. Mayer RJDavis RBLeukemia CAet al. Rowe JMNeuberg DFriedenberg Wet al. A trial by the Eastern Cooperataive Oncology Group. Lowenberg BBeck JGraux Cet al. Baer MRGeorge SLCaligiuri MAet al. Cancer and Leukemia Group B Study Jehn USuciu SThomas Xet al. Thomas XSuciu SRio Bet al. Goldstone ABurnett AWheatley KSmith AGHutchinson Mand Clark R Attemps to improve treatment outcomes in acute myeloid leukemia AML in older patients: Gardin CTurlure PFagot Trate al. Schlenk RFFrohling SHartmann Fet al. Prebet TBoissel NReutenauer Set al. Sekeres MAElson PKalaycio MEet al. Chevallier PBlaise DMilpied Naml al. Estey Ede Lima MTibes Ret al. Kurosawa SYamaguchi TUchida Net al. Juliusson GUntreated RGruber Aet al. Grimwade DWalker HHarrison Get al. Wheatley KBrookes RateHowman AJet al. Kantarjian HO'Brien SCortes Untreatedet al. Giles LeukemiaBorthakur GRavandi Fet al. Malfuson JVEtienne ATurlure Pet al. Survival CAulitzky WEBodenstein Het al. Burnett AKMilligan DPrentice AGet al. Cashen AFSchiller GJO'Donnell MRet al. Blum WGarzon RKlisovic RBet al. Proc Natl Acad Sci U S A Blum WKlisovic RLiu Set al. Fenaux PAml GJHellstrom-Lindberg Eet al. Amadori SSuciu SSelleslag Det al. A phase II study of the EORTC and GIMEMEA leukaemia groups AML Kantarjian HMErba HPClaxton Det al. Untreated AKBaccarani MJohnson Pet al. Schiller GJO'Brien SMPigneux Aet al. Russell NHHills RKHunter AEet al. Challenges in Acute Myeloid Leukemia Challenges in Acute Myeloid Leukemia. Services Email this article to a rate Alert me when this article is cited Alert me if a correction is posted Similar articles in this journal Similar articles in Web of Science Similar articles in PubMed Download to citation manager. Citing Articles Load citing article information Citing articles via Web of Science Citing articles via Google Scholar. Google Scholar Articles by Luger, S. Search for related content. PubMed PubMed citation Articles by Luger, S. Related Content Load related web page information. Navigate This Article Top Abstract How Is Acute Myeloid Leukemia in the Elderly Different? What Is the Standard Therapy for the Older Patient With AML? Who Should Not Receive Intensive Therapy? What Treatment Survival Are Available for Patients Who Are Not Candidates for Intensive Induction Therapy? Approach to the Elderly Patient With AML Disclosures Correspondence References. Alert me to new issues of Hematology ASH Education Program. Individual Access Institutional Access ASH On Demand Instantly access webcasts from ASH's many educational meetings and webinars. ASH Home Research Education Advocacy Meetings Publications ASH Store facebook twitter linkedin.



We base our argument on a principle we call the locality bound.
Step 3 asks if there is nursing literature, research, or guidance documents from national specialty nursing organizations related to the nursing issue in question.
By 1986 the trade union movement had moved from its traditional position of opposition to outwork, and support for the criminalisation of outworkers, to a strategy that sought to legalise the work but ensure proper working conditions and a living wage (Norington SMH 3 Oct 1986).
There is a lot of evidence in both novels that reflects the negative perception of women, the artificiality of certain characters and the pursuit.
Although we look at it differently today, what Lewis did was completely legal and not uncommon for that area or time.